Osmosis/Osmotic Pressure/Vant Hoff Equation/Solutions/Explanation in Tamil/TN11th Std/CBSE 12 - YouTube
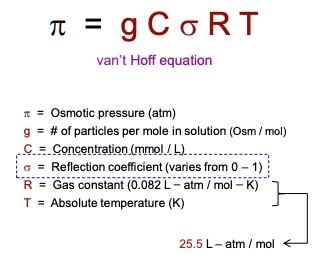
SOLVED: TC = g C o RT van't Hoff equation Osmotic pressure (atm) of particles per mole solution (Osm mol) Concentration(mmol Reflection coefficient (vares from Gas constant 082 atm mol Absolute temperature (
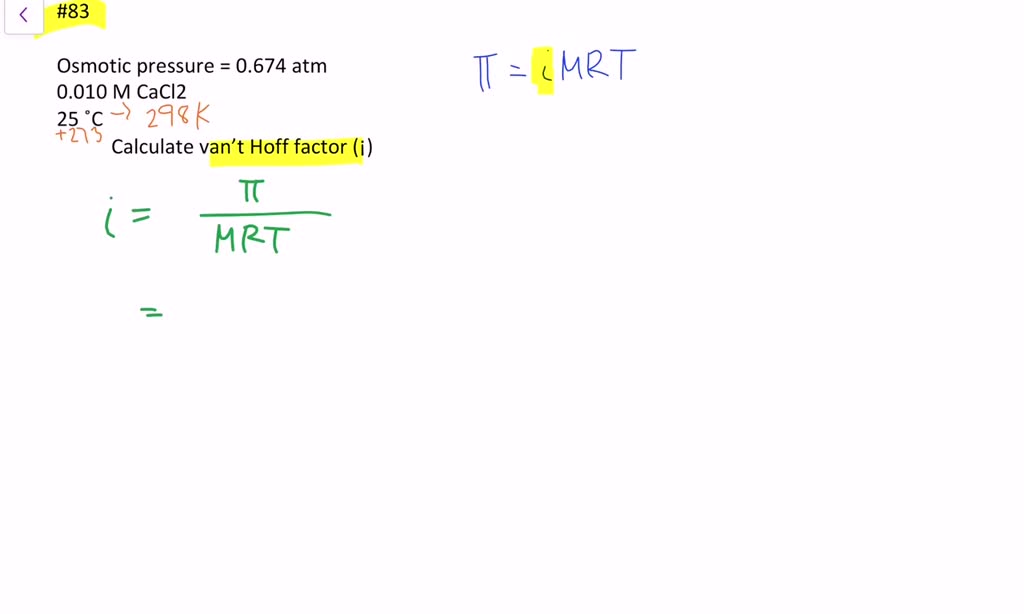
SOLVED:The osmotic pressure of a 0.010M aqueous solution of CaCl2 is found to be 0.674 atm at 25^∘ C . Calculate the van't Hoff factor, i, for the solution.
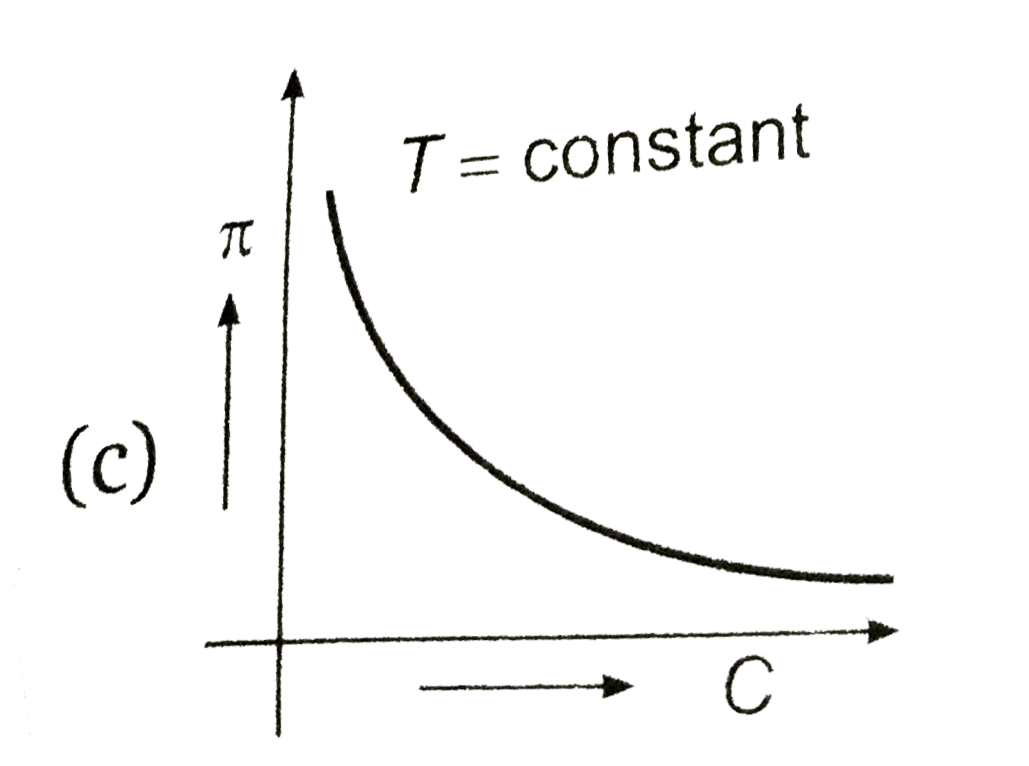
van't Hoff proved that osmotic pressure (pi) is a colligative property. For an ideal solution, osmotic pressure(pi) is helpful to determine that molecular mass of solute using M(B)=(W(B)RT)/(pi.V) Relation can exxpressed by

vant hoff equation for osmotic pressure of solution and expiration of degree of dissolution - Chemistry - - 14038313 | Meritnation.com

The observed osmotic pressure for a 0.10M solution of Fe (NH4)2 (SO4)2 at 25^o C is 10.8 atm. The expected and experimental (observed) values of Van't Hoff factor (i) will be respectively: (